Microsegmentation Buyer's Guide and Checklist for BioTech and Pharma
A comprehensive, vendor-neutral guide to evaluating microsegmentation solutions that protect intellectual property, clinical data, and lab equipment while meeting FDA 21 CFR Part 11 and IEC 62443 compliance requirements.
Download the Guide


Get Your Comprehensive Network Segmentation Buyer's Guide
This vendor-neutral pharmaceutical cybersecurity solutions guide provides biotech and pharma security architects with practical frameworks, technical criteria, and evaluation tools needed to select the right microsegmentation solution for protecting intellectual property and research networks. Based on industry research and real-world implementations, it delivers objective insights to help you navigate complex vendor landscapes and make informed decisions.
- Technical Evaluation Checklists for Pharmaceutical
- 50+ Critical Vendor Assessment Questions
- ROI and Implementation Planning Framework


‟Now we can plug any device into the network. It gets profiled along with the identity behind that device, and no matter where that device plugs into the network, the policy follows.”
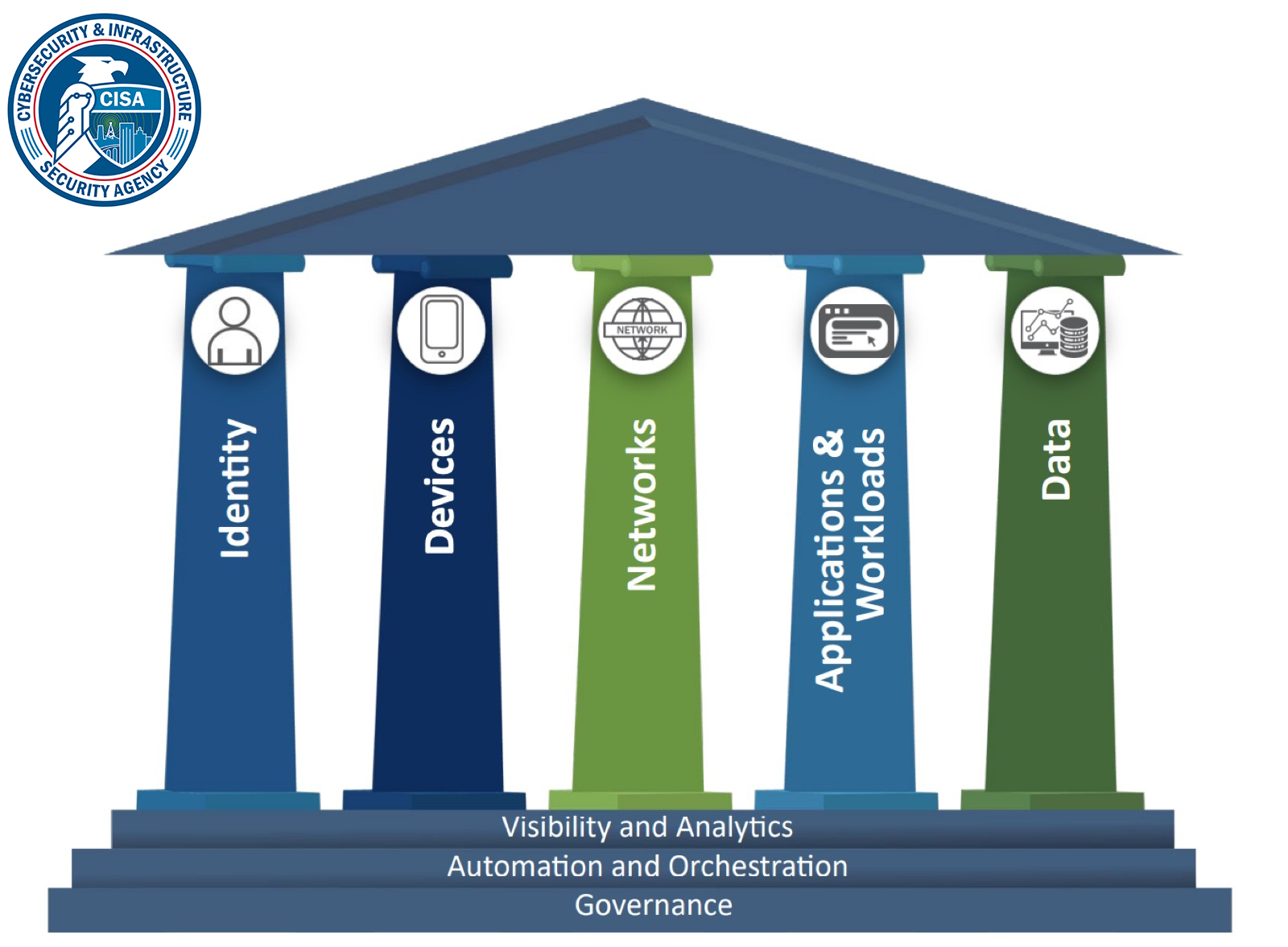
What is Microsegmentation?
Identity-based microsegmentation enables biotech and pharmaceutical companies to rapidly secure research and manufacturing networks by applying granular, context-aware policies that protect intellectual property, clinical data, and lab equipment. By automatically controlling access across IT, IoT, and OT environments, organizations prevent lateral movement attacks while eliminating the need for new hardware, agents, or complex network reconfigurations.
Why Modern Microsegmentation is Critical for BioTech and Pharmaceutical Organizations Today
- Ransomware attacks target healthcare's lateral pathways—67% of healthcare organizations were hit in 2024, with attackers moving from compromised workstations to critical medical systems.
- The expanded attack surface from lab instruments, manufacturing OT systems, and interconnected research networks creates numerous potential entry points that can't be properly secured with traditional tools.
- Pharmaceutical regulations like FDA 21 CFR Part 11, IEC 62443, and emerging OT cybersecurity guidance now specifically mandate comprehensive network segmentation for protecting intellectual property and maintaining production integrity.
- Pharmaceutical breaches can cost billions in lost research investment through intellectual property theft, making microsegmentation essential to contain breaches and limit lateral movement.
Essential Checklists and Assessment Tools Included
- Reduce Attack Surface: Comprehensive technical requirements checklist covers must-have capabilities including lab equipment discovery and GxP-compliant policy management to reduce vulnerable attack paths by 70%-90%.
- Cut Operational Costs: 50+ vendor evaluation questions organized by critical categories like research network visibility and manufacturing system support help achieve 60%-80% reduction in operational overhead.
- Faster Medical System Recovery: Decrease mean-time-to-contain from 4-6 hours to under 10 minutes, critical for maintaining patient safety during incidents.
- Streamline Compliance: Implementation planning templates with phased deployment timelines and resource allocation guidelines deliver 50% less audit preparation time for FDA and IEC 62443 requirements.
- Prevent Lateral Movement: GSK case study demonstrates real-world protection against attacks that typically compromise drug research and production systems for an average of 280 days before detection.



